How do you find the lowest energy of a chair conformation?
1 Answer
I take it you are talking about substituted and disubstituted cyclohexanes.
Explanation:
Cyclohexanes undergo fairly facile conformational exchange where axial substituents are exchanged for equatorial substituents and vice versa. There is no substitute for the use of models. And models will always be permitted in examinations.
Now not only do you have to be able to use the model to predict the preferred conformation; you also have to be able to depict that conformational change on paper. This is not so trivial, and takes some practice. With a simple ring flip, equatorial positions become axial, and vice versa.
Even the simplest such example for cyclohexane, a handwritten formula showing the exchange of axial for equatorial substituents can be troublesome.
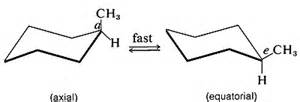
Knowing nothing else (and believe me I do not mean to insult your intelligence), which of the cyclohexane conformations above would you predict to be the most stable? Why? Please post the answer here.

