Because there exist exactly three #p# orbitals per quantum level #n#, and because the #(n-1)d# orbital is closer in energy to the #(n-1)s# orbital than an #np# orbital.
It sounds like a misunderstanding of the hybridization notation.
HYBRIDIZATION NOTATION
Having #sp^3# hybridization tells you that one #ns# orbital mixes with three #np# orbitals to generate four identical (in symmetry), degenerate (in energy) #sp^3# orbitals. That way, the given compound can form up to four identical bonds if it wishes.
From the explanation above, using #n = 4#, it follows that #sp^4# implies the existence of a fourth #(n-1)p# orbital, or implies an #np# orbital somehow participates as the fourth #p# orbital.
However, that is not reasonable, given that an #np# orbital is too far away in energy to interact in place of using the #(n-1)d# orbital for #n = 4#. Furthermore, there exist only three #(n-1)p# orbitals, not four---remember that for #l = 1# (for all #p# orbitals), #m_l = {-1,0,+1}#.
A MORE EXPLICIT EXAMPLE
The #3d# orbital, for instance, is closer in energy to the #3s# orbital than the #4p# is to the #3s# for a given atom.
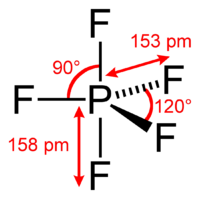
That means it is more favorable to mix one #3s#, three #3p#, and one #3d# if the compound wishes to form five bonds using #sp^3d# hybridization.
An example of #sp^3d# would be #PF_5#.
 )
)
 )
) 
