Why does furan undergo Diels Alder reaction?
1 Answer
Dec 27, 2016
Furan undergoes Diels-Alder reactions because it can behave as a dienophile.
Explanation:
Furan is classed as an aromatic compound because a lone pair on the oxygen can be delocalized into the ring to give a planar, cyclic, 6π conjugated system.
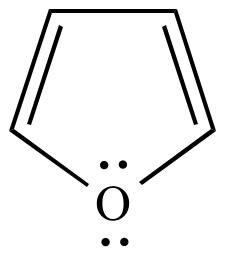
However, an
The resonance stabilization of furan is only 67 kJ/mol compared to 150 kJ/mol for benzene.
The aromatic π system of furan can be disrupted if a process is energetically favourable.
Thus, furan readily undergoes Diels-Alder reactions and reacts as a diene in the presence of a dienophile.


