Why do solutes dissolve in solvents?
1 Answer
The major factor that determines whether solutes dissolve in solvents is entropy.
To form a solution we must:
1. Separate the particles of the solvent.
2. Separate the particles of the solute.
3. Mix the particles of solvent and solute.
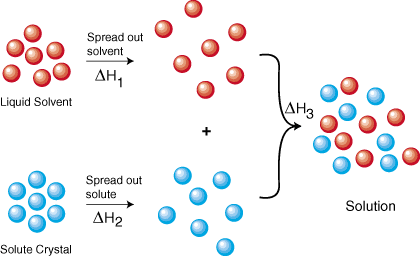
For the solution process to be favourable,
Nonpolar Solvent – Nonpolar solute
If both solvent and solute are nonpolar, all the
Polar Solvent – Polar Solute
If both solvent and solute are polar, all the
LIKE DISSOLVES LIKE.
Polar Solvent – Nonpolar Solute
If a nonpolar solute such as oil mixes with a polar solvent like water,


