Why are ionic server soluble even though the force between them is very strong but covalent compounds which have less force between the molecules are not soluble why ?.
1 Answer
Explanation:
The nature of water
Water is a highly polar substance.
Its
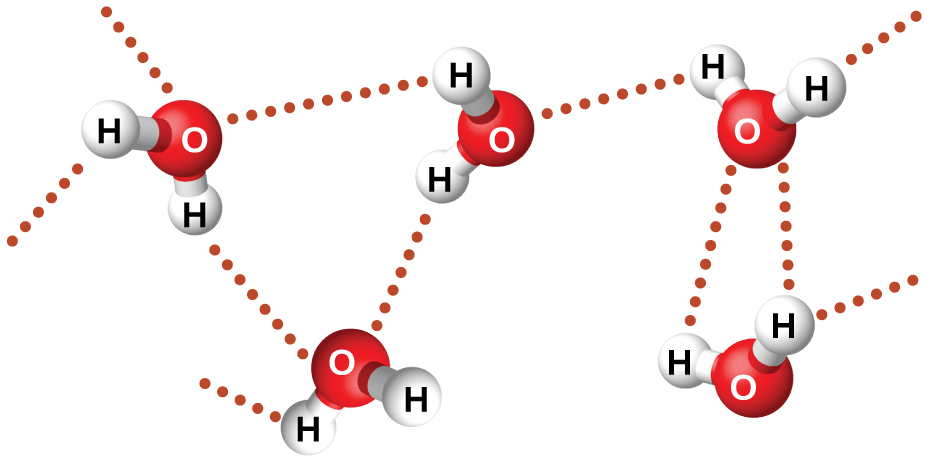
Water molecules are strongly attracted to each other by these hydrogen bonds.
Nonpolar solutes
The intermolecular forces in many covalent molecules are relatively weak London dispersion forces and dipole-dipole interactions.
Thus, these molecules can easily separate from each other.
But the water molecules are so strongly attracted to each other that the solute molecules cannot get between them.
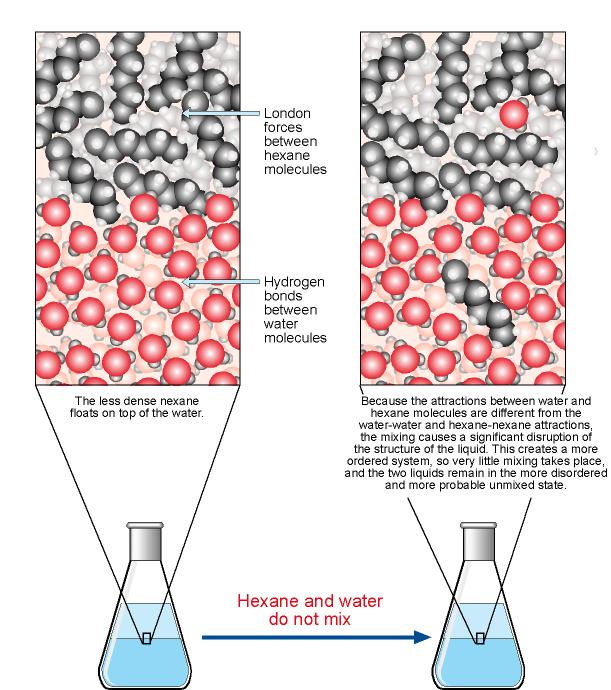
The mixture will separate into two phases.
The particles in an ionic compound like
However, the attractions between the ions and the highly polar water molecules are roughly the same size.
Energetic water molecules can dislodge ions from the surface of the crystal.
Once these ions are in the aqueous phase, they become surrounded by a **solvation shell'' of water molecules.
This shell reduces the attraction between the ions and helps to prevent them from going back to the crystal surface.
Note: The ion-ion attractions in some salts are so strong that water can't overcome them.
For example,

