Which is an Arrhenius Acid?
#H_2SO_4#
#LiOH#
#NH_2CH_3#
#CH_3CH_3#
1 Answer
Aug 27, 2016
Explanation:
Sulfuric acid (
(
By definition an Arrhenius acid is any substance that generates hydrogen ions in aqueous solution.
Here's an example of a general Arrhenius acid:
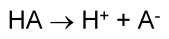
#HA# is an acid because it dissociates in water to produce hydrogen ions#H^(+)# and the base#A^(-)# .
However, LiOH is not an Arrhenius acid because it is a strong base and it dissociates almost completely in solution; it has the ability to produce hydroxide ions in solution:
-
#CH_3NH_2# (methylamine) is a base and it cannot give off hydrogen ions. -
#CH_3CH_3# (ethane) is a gaseous hydrocarbon and it will not give off hydrogen ions.

