What stereoisomers are obtained from hydroboration–oxidation of 1-ethylcyclohexene?
1 Answer
Dec 5, 2014
You get a racemic mixture of 2-ethylcylohexanol enantiomers.
Explanation:
You get a racemic mixture of 2-ethylcylohexanol enantiomers.
Hydroboration-oxidation is a method of making alcohols from alkenes. For example,
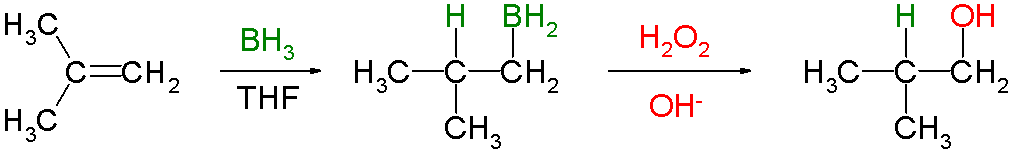
The overall reaction amounts to an "anti-Markovnikov" addition of H-OH to the C=C bond.
Also, the reaction has syn stereochemistry, because the H-BH₂ adds to the same side of the alkene.
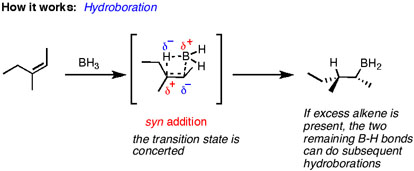
With 1-ethylcyclohexene, the addition of BH₃ gives a pair of enantiomers.
Product 1 is (1 S,2 R)-2-ethylcyclohexanol.
Product 2 is ( 1R,2 S)-2-ethylcyclohexanol.
Here’s a video on the hydroboration/oxidation of alkenes.

