What is the dipole moment of water?
1 Answer
Oct 22, 2015
The dipole moment of water is
Explanation:
The dipole moment arises because oxygen is more electronegative than hydrogen; the oxygen pulls in the shared electrons and increases the electron density around itself.
This creates an electric dipole moment vector, with the partial negative charge on the oxygen atom.
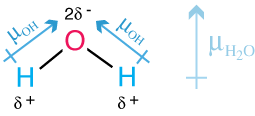
There are two


