What is K-electron capture?
1 Answer
Sep 17, 2015
K-electron capture is the capture of a
Explanation:
Electron capture occurs when the nucleus of an unstable isotope captures an inner-orbital electron.
In the process, a proton combines with the electron and forms a neutron, and an X-ray is released in the process.
The atomic number decreases by one unit, but the mass number remains unchanged.
The captured electron usually comes from the
If the electron comes from the
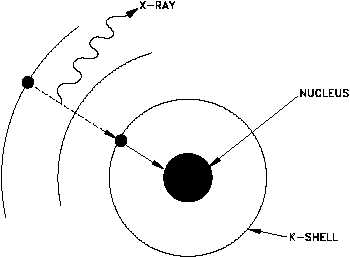
Capture from the

