What is bond line notation?
1 Answer
Dec 2, 2014
Bond line notation is a shorthand way of writing organic structural formulas:
Explanation:
- The carbon atoms and the hydrogen atoms attached to them are not shown.
- Only the bonds between the carbon atoms are shown as lines.
- The vertices and end of lines represent the carbon atoms.
- Any unfilled valences on carbon are assumed to be filled by hydrogen atoms.
- All atoms other than carbon, plus any hydrogen atoms attached to them, are shown.
For example, the bond line notation for propane is

In the above diagram, the vertex and the ends of the lines represent the carbon atoms.
The two end C atoms each contain three H atoms, since they have each formed one bond to the middle C atom.
The middle C contains only two H atoms, since it has already formed two bonds with the other carbons.
Some more examples are shown below.
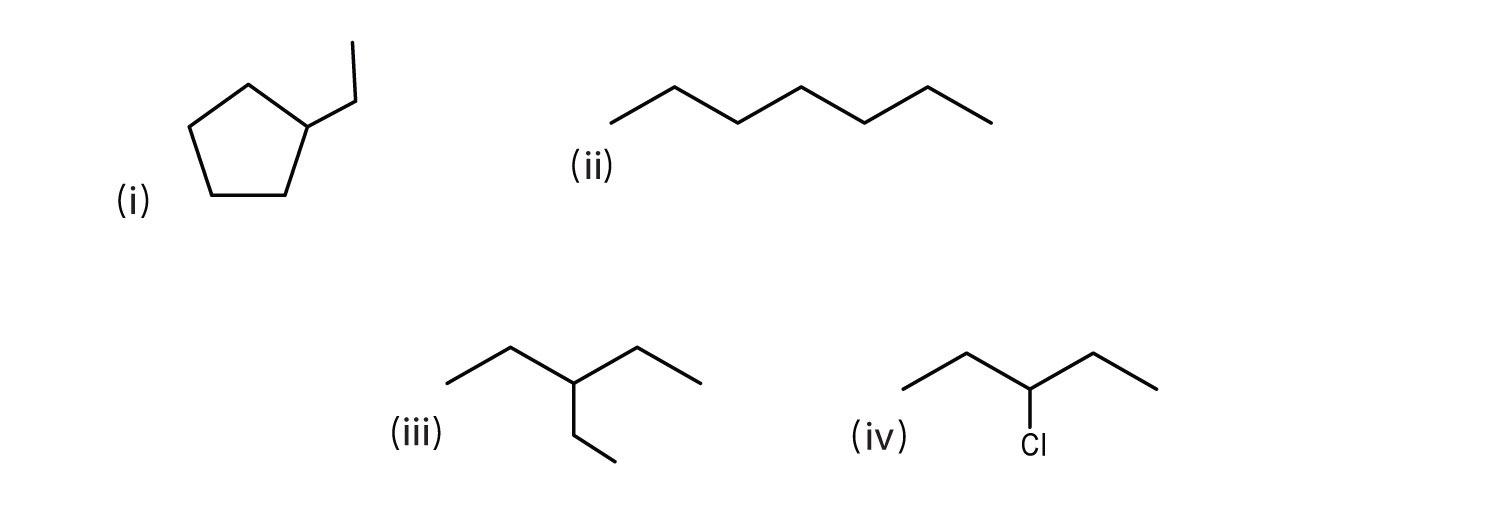
(i) ethylcyclopentane
(ii) heptane
(iii) 3-ethylpentane
(iv) 3-chloropentane
Here are still more bond line structures.

Note that we must show the H atoms that are attached to O and N atoms.
Here’s a video on bond line notation.

