What is an example of an antibonding orbitals practice problem?
1 Answer
Feb 11, 2016
Practice reading the MO diagram of
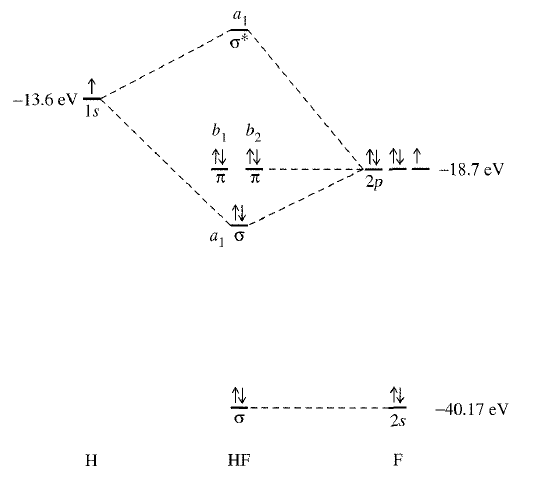
From the diagram:
- Identify the antibonding molecular orbital.
- Identify the three nonbonding molecular orbitals.
- Identify the only bonding molecular orbital.
- Which orbitals correspond to fluorine's three lone pairs of electrons?
- What is the bond order? Does it make sense given the single bond in
#"H"-"F"# ? - Why is there one
#sigma# orbital by itself at the bottom? What does that suggest about the energies of the#2s# of#"F"# and the#1s# of#"H"# ?

