What identifying characteristics would be present in the mass spectrum of a compound containing two bromine atoms?
1 Answer
There should be M, M+2, and M+4 peaks with relative intensities of 1:2:1.
Explanation:
For example, see the mass spectrum of 1,3-dibromopropane.
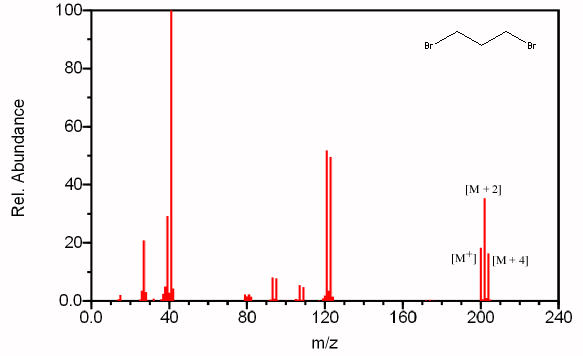
Natural bromine is a mixture of 50.50 %
Thus, the molecule could contain two
This will give rise to three molecular ion peaks, each separated by two mass units.
We can calculate the relative size of each peak.
M peak:
Probability of
M+2 peak:
Probability of
M+4 peak:
Probability of
If we set the relative probability of the largest peak at 100, the relative intensities become
Other feature:
You should see a peak at M-79 (loss of
Compare the peak at 121 in the spectrum of 1,3-dibromopropane,

