What happens when alkenes are oxidized?
1 Answer
Alkenes are oxidised to give carbonyl compounds or carboxylic acids depending upon the condition.
Explanation:
So ozonolysis is an example of oxidative cleavage reaction that leads to the breaking
- Oxidative ozonolysis
- Reductive ozonolysis
Let me begin with oxidative ozonolysis. In this reaction,
 )
)
Now taking the second condition, i.e reductive ozonolysis.
In this case, oxygen is oxidized to intermediate form i.e carbonyl compound. The reductive workup is done with
![https://www.masterorganicchemistry.com/2013/04/23/alkene-reactions-ozonolysis/]
(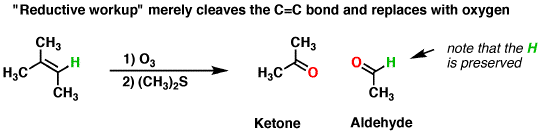 )
)
Hope it helps!!


