What does hydroboration–oxidation of a terminal alkyne form?
1 Answer
Oct 26, 2014
Hydroboration-oxidation of a terminal alkyne forms an aldehyde.
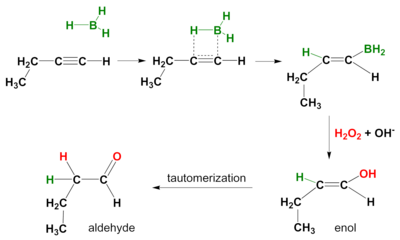
The reaction takes place in two steps.
Step 1 is the syn addition of a B-H bond across a π bond in the alkyne.
Instead of BH₃, we use a bulky borane like disiamylborane or 9-borabicyclo[3.3.1]nonane (BBN).
This increases the proportion of addition to the less substituted carbon atom and prevents hydroboration across both π bonds,
In Step 2, the organoborane is oxidized with alkaline hydrogen peroxide.
The initial product is an enol, which rapidly tautomerizes to an aldehyde.
Hydroboration-oxidation converts a terminal alkyne into an aldehyde with the same number of carbon atoms.
For example, but-1-yne is converted to butanal.


