What chemical starts off the chain reaction in the initiation step of an anti-markovnikov radical addition?
1 Answer
The initiator in a free radical addition reaction is a substance that decomposes into free radicals under mild conditions.
Explanation:
An initiator should have bonds with low dissociation energies (e.g.
Common initiators are:
Azo compounds
Azo compounds (
AIBN (azo-bis-isobutyronitrile) is a convenient free radical initiator because it decomposes at relatively low temperatures.
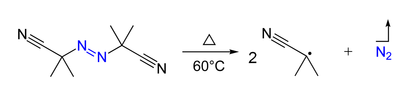
(from en.wikipedia.org)
Organic peroxides
Two common peroxide initiators are
(a) Di-t-butyl peroxide
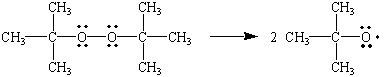
(from chemed.chem.purdue.edu)
(b) Benzoyl peroxide
(from research.cm.utexas.edu)

