What are the three allotropes of carbon? Also explain their internal structures.
1 Answer
There are more than three allotropes of carbon. These include diamond, graphite, graphene, carbon nanotubes, fullerenes, and carbon nanobuds.
Diamond
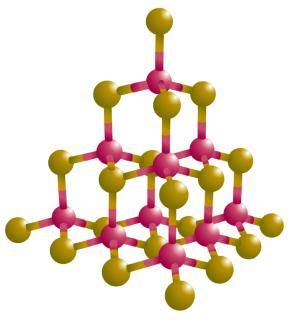
Each carbon atom in a diamond is covalently bonded to four other carbons in a three-dimensional array. A diamond is essentially one giant molecule.
Graphite

In graphite, the carbon atoms are joined in sheets of linked hexagons that look like chicken wire. Each sheet is essentially a single molecule.
Within a sheet, each carbon atom forms strong covalent bonds to three other carbon atoms. The stacked sheets are held together only by weak intramolecular forces.
Graphene

Graphene is pure carbon in the form of a single sheet of graphite that is just one atom thick.
Carbon Nanotubes
A carbon nanotube is like a sheet of graphene rolled into a cylindrical tube of carbon atoms. Each atom bonded to three other atoms, and the tube is one atom thick.
Buckminsterfullerene, C₆₀

Buckminsterfullerene consists of a single sheet of carbon atoms wrapped into a sphere. Each carbon atom is bonded to three other atoms. Sixty carbon atoms form the shape of a ball with a carbon atom at each corner of 20 hexagons and 12 pentagons.
Many other balls of carbon are known, including C₇₀, C₇₆, C₈₄ and C₅₄₀. They contain various numbers of pentagons and hexagons and are known collectively as "buckyballs" or "fullerenes".
Carbon Nanobuds
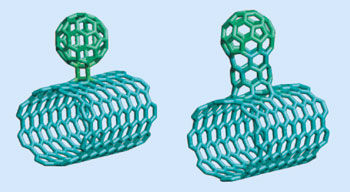
Carbon nanobuds are an allotrope of carbon in which fullerene-like "buds" are covalently attached to the outer sidewalls of carbon nanotubes.

