What are the reactions for amines and amides?
2 Answers
An amine is an organic compound, related to ammonia, that contains a nitrogen atom bonded to one or more alkyl groups on each molecule




Nitrogen atoms that are part of an aromatic ring have planar configuration(sp2 configuration ) and not stereogenic centres.Because of aromacity amines in aromatic rings are stable.
As the hybridation of the nitrogen its effects its power as an acid and base.
Higher s character of the nitrogen makes the compound acidic for example in pyridine and nitriles.
Secondly, aniline and p-nitroaniline are weaker bases due to delocalization of the nitrogen non-bonding electron pair into the aromatic ring (and the nitro substituent). This is the same delocalization that results in activation of a benzene ring toward electrophilic substitution.
The following resonance equations, which are similar to those used to explain the enhanced acidity of ortho and para-nitrophenols illustrate electron pair delocalization in p-nitroaniline.
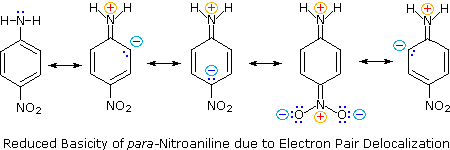
Although resonance delocalization generally reduces the basicity of amines, a dramatic example of the reverse effect is found in the compound guanidine (pKa = 13.6).
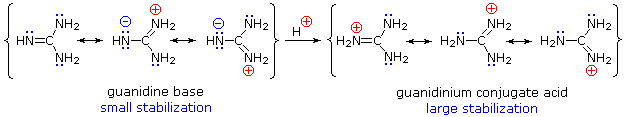
Amines are mostly basic but some have reduced basicity because of these reasons
When reacted with acids, amines donate electrons to form ammonium salts.

Acid halides react with amines to form substituted amides

Production of Schiff bases from primary amines
Aldehydes and ketones react with primary amines to give a reaction product (a carbinolamine) that dehydrates to yield aldimines and ketimines (Schiff bases)

If you react secondary amines with aldehydes or ketones, enamines form.

Amines react with sulfonyl chlorides to produce sulfonamides. A typical example is the reaction of benzene sulfonyl chloride with aniline.

The Hinsberg test
The Hinsberg test, which can distinguish primary, secondary, and tertiary amines, is based upon sulfonamide formation. In the Hinsberg test, an amine is reacted with benzene sulfonyl chloride. If a product forms, the amine is either a primary or secondary amine, because tertiary amines do not form stable sulfonamides. If the sulfonamide that forms dissolves in aqueous sodium hydroxide solution, it is a primary amine. If the sulfonamide is insoluble in aqueous sodium hydroxide, it is a secondary amine. The sulfonamide of a primary amine is soluble in an aqueous base because it still possesses an acidic hydrogen on the nitrogen, which can be lost to form a sodium salt.

Oxidation of amines
Although you can oxidize all amines, only tertiary amines give easily isolated products. The oxidation of a tertiary amine leads to the formation of an amine oxide.

Arylamines tend to be easily oxidized, with oxidation occurring on the amine group as well as in the ring.
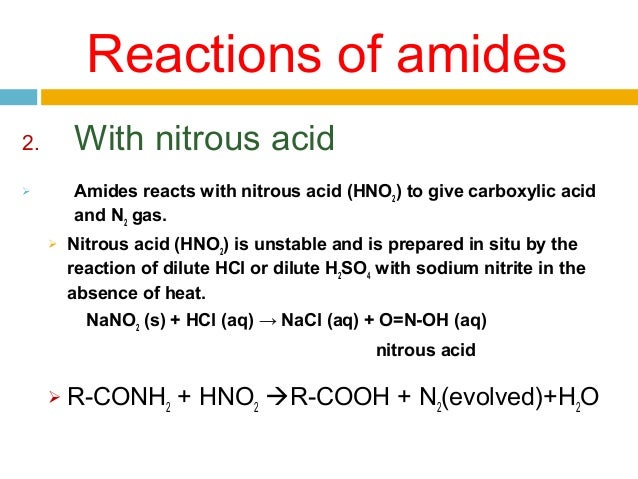
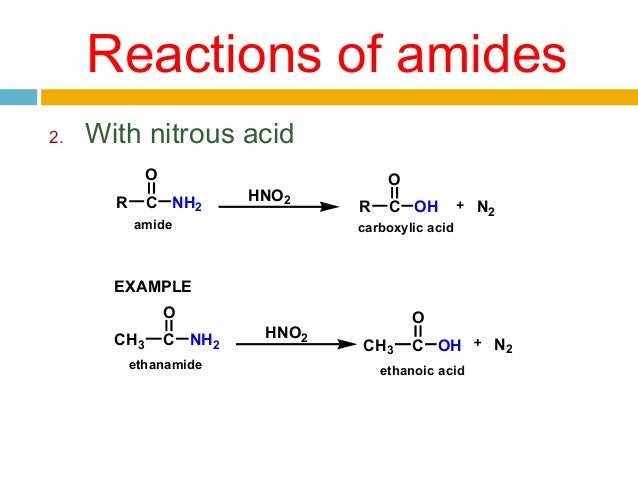
Reaction with nitrous acid
Nitrous acid is unstable and must be prepared in the reaction solution by mixing sodium nitrite with acid.
Primary amines react with nitrous acid to yield a diazonium salt, which is highly unstable and degradates into a carbocation that is capable of reaction with any nucleophile in solution. Therefore, reacting primary amines with nitrous acid leads to a mixture of alcohol, alkenes, and alkyl halides.

Primary aromatic amines form stable diazonium salts at zero degrees.

Secondary aliphatic and aromatic amines form nitrosoamine with nitrous acid.

Tertiary amines react with nitrous acid to form N‐nitrosoammonium compounds.

Reactions of aromatic diazonium salts
Diazonium salts of aromatic amines are very useful as intermediates to other compounds. Because aromatic diazonium salts are only stable at very low temperatures (zero degrees and below), warming these salts initiates decomposition into highly reactive cations. These cations can react with any anion present in solution to form a variety of compounds. The following figure illustrates the diversity of the reactions.

I'll write about amides in another answer to this question
Amides can undergo some special reaction or types of reaction which are the following ones
1) Nucleophilic Acyl Subsctitution
2) Reduction of amides with LiAl4
3) Reaction with nitrous acid
4) Hoffman Degradation
5) Dehydration of Amides
Nucleophilic Acyl Subsctitution

Heat helps in dissociation of water
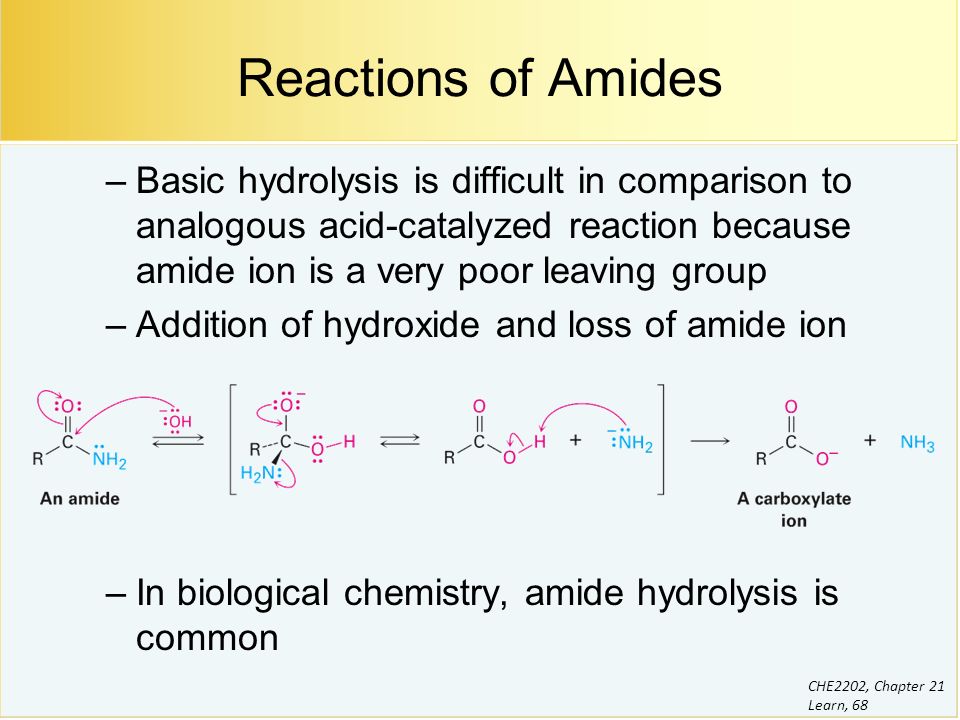
Mechanism
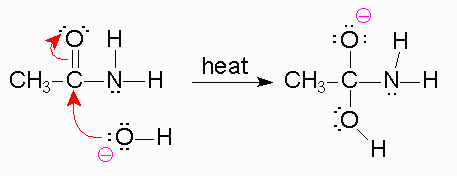
To begin, we know that in the first step, a strong base is going to be used like
Now, when the bond forms between the
 )
)
*The reason the amine is a poor leaving group is because the amine is a strong base, and strong bases are very unstable. Leaving groups are based off their stability in solution, like the halides, tosylate,
 )
)
Mechanism of acid catalysed amide hydrolysis
Step 1)An acid/base reaction. Since we only have a weak nucleophile and a poor electrophile we need to activate the ester. Protonation of the amide carbonyl makes it more electrophilic
Step 2:
The water O functions as the nucleophile attacking the electrophilic C in the C=O, with the electrons moving towards the oxonium ion, creating the tetrahedral intermediate.
Step 3:
An acid/base reaction. Deprotonate the oxygen that came from the water molecule.
Step 4:
An acid/base reaction. Need to make the -NH2 leave, but need to convert it into a good leaving group first by protonation.
Step 5:
Use the electrons of an adjacent oxygen to help "push out" the leaving group, a neutral ammonia molecule.
Step 6:
An acid/base reaction. Deprotonation of the oxonium ion reveals the carbonyl in the carboxylic acid product and regenerates the acid catalyst.

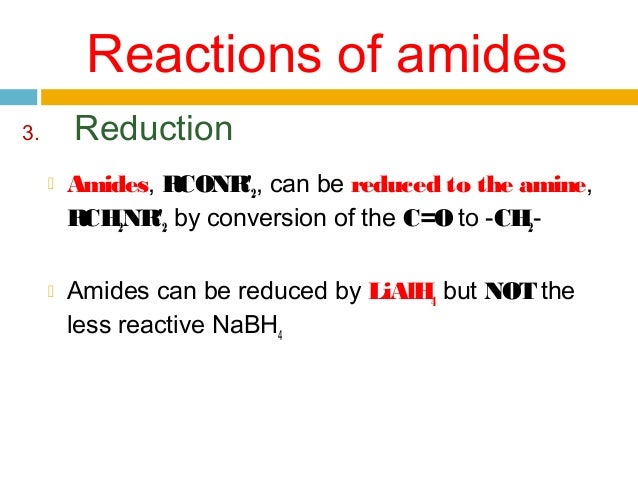
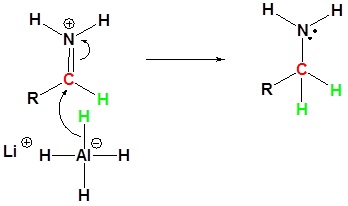
Reduction of amides with LiAl4
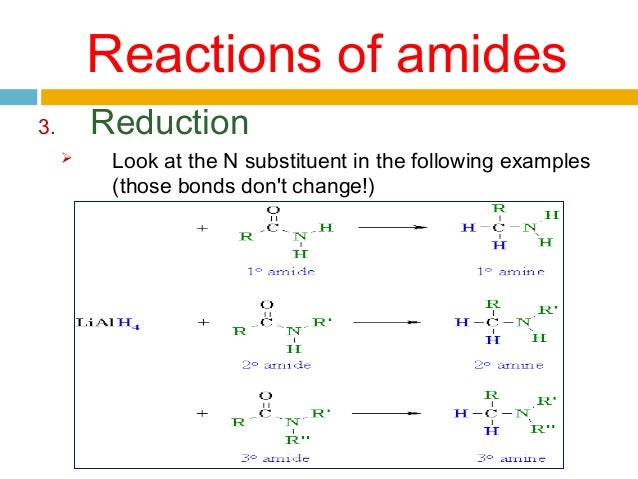
Nucleophilic addition by the hydride
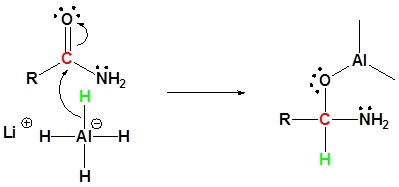
Leaving group removal
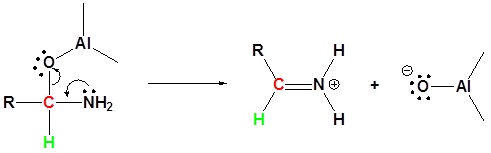
Nucleophilic attach by the hydride
Hoffman Degradation
Hoffman Degradation is a reaction between an amide and a mixture of bromine and sodium hydroxide solution.Heat is needed.
Mechanism
Step 1) Base abstracts an acidic N-H proton, yielding an anion
Step 2) The anion reacts with bromine in an α-substitution reaction to give an N-bromoamide.
Step 3) Base abstraction of the remaining amide proton gives a bromoamide anion.
Step 4) The bromoamide anion rearranges as the R group attached to the carbonyl carbon migrates to nitrogen at the same time the bromide ion leaves, giving an isocyanate.
Step 5) The isocyanate adds water in a nucleophilic addition step to yield a carbamic acid (aka urethane).
Step 6) The carbamic acid spontaneously loses CO2, yielding the amine product.

Dehydration of amides
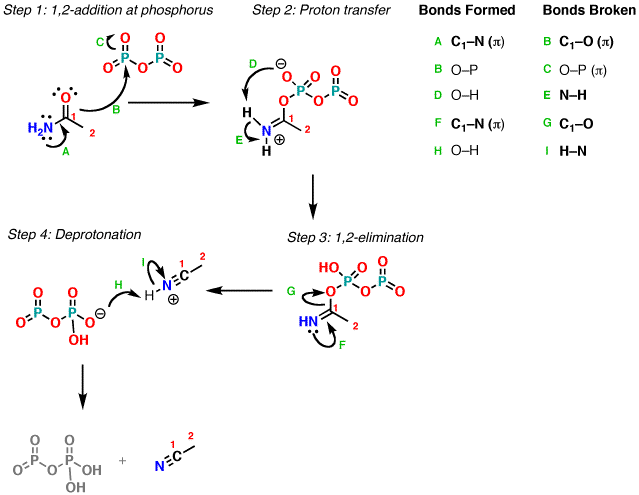
Amines are dehydrated by reacting it with phosphorus(V) oxide or phosphorus oxychloride and heating the mixture

