What are stereoisomers? Give me an example
1 Answer
What are stereoisomers? Give me an example
Stereoisomers are molecules that have the same molecular formula and sequence of bonded atoms, but differ in the three-dimensional orientations of their atoms in space.
There are two kinds of stereoisomers: geometric and optical.
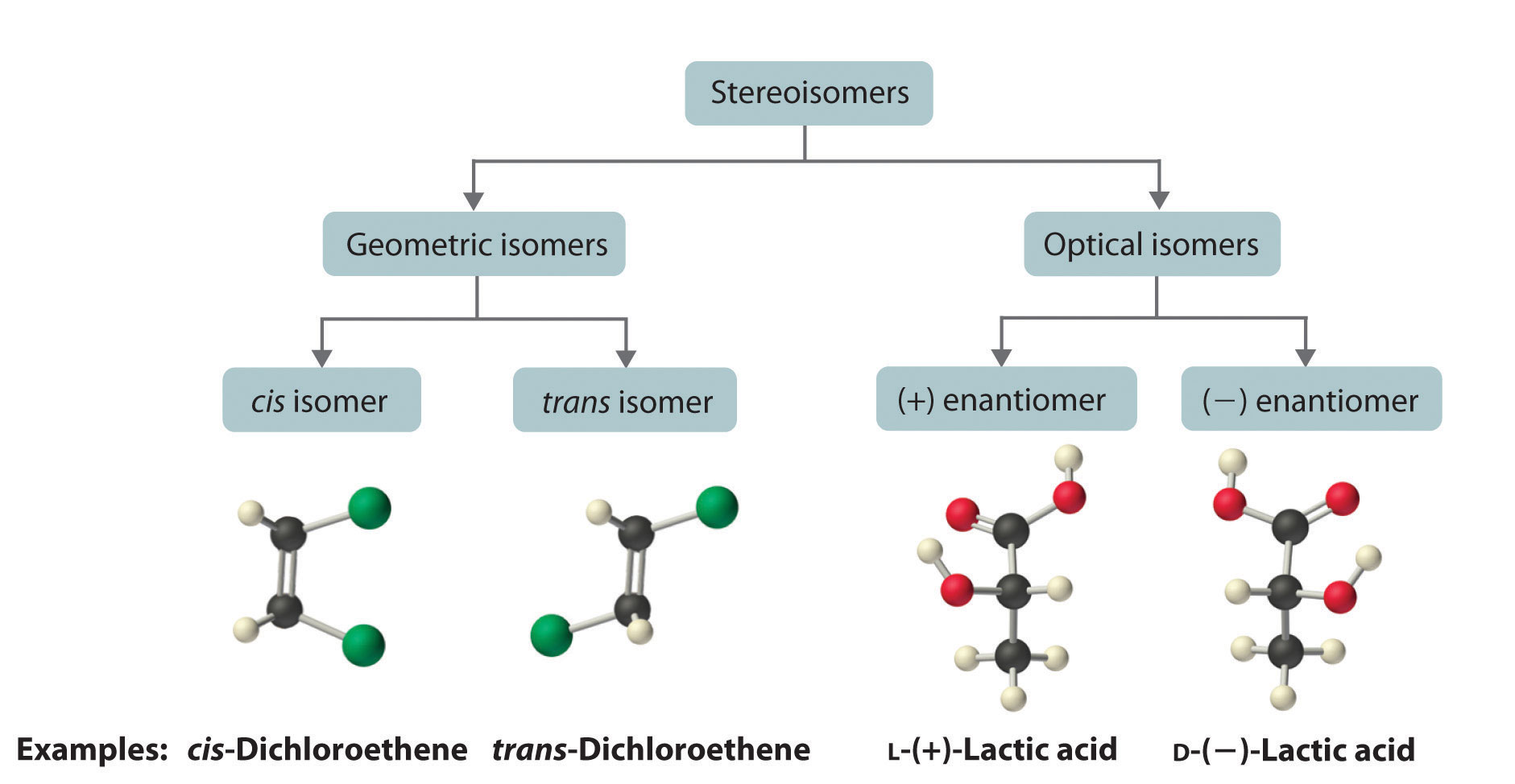
Geometric Isomers
Geometric isomers differ in the relative positions of substituents in a rigid molecule.
For example, 1,2-dichloroethene can exist as the cis isomer, with both Cl atoms on the same side of the double bond, or as the trans isomer, with the Cl atoms on opposite sides of the double bond.
Cis and trans isomers have different physical and chemical properties.
Optical Isomers
Optical isomers are molecules whose structures are mirror images that cannot be superimposed on one another in any orientation.
Such molecules are said to be chiral.
For example, D-lactic acid and L-lactic acid are optical isomers.
Optical isomers differ only in their interactions with polarized light and with other chiral molecules.

