What are metal activity series?
1 Answer
An activity series is a list of substances ranked in order of relative reactivity.
For example, magnesium metal can displace hydrogen ions from solution. So magnesium is more reactive than hydrogen:
Mg(s) + 2H⁺(aq) → H₂(g) + Mg²⁺(aq)
Zinc metal can also displace hydrogen ions from solution:
Zn(s) + 2H⁺(aq) → H₂(g) + Zn²⁺(aq)
so zinc is also more active than hydrogen.
But magnesium metal can displace zinc ions from solution:
Mg(s) + Zn²⁺(aq) → Zn(s) + Mg²⁺(aq)
so magnesium is more active than zinc.
The activity series including these elements would be Mg > Zn > H.
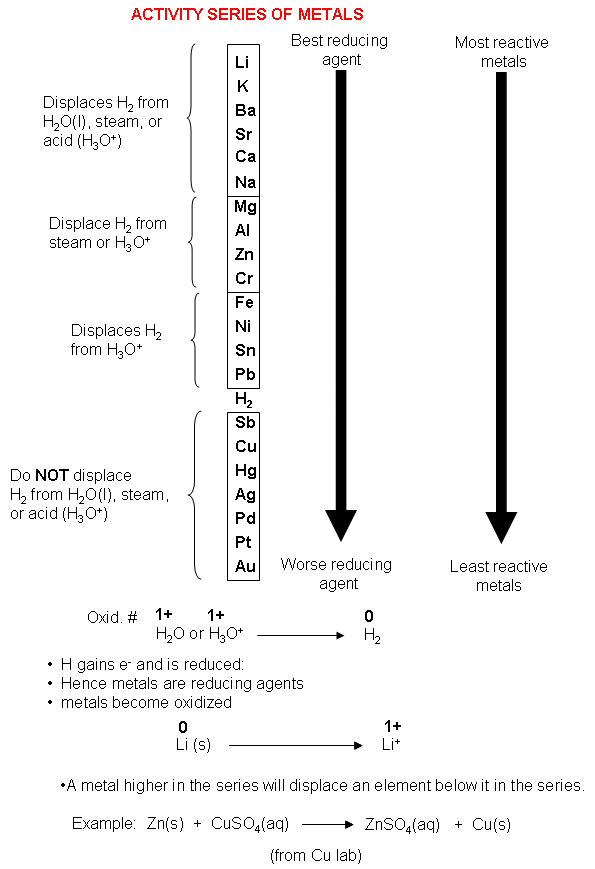
Chemists have built up a complete activity series in a similar way.
The most active metals are at the top of the table; the least active are at the bottom.
Any metal that is higher in the series will displace a metal that is below it in a single displacement reaction.
In the language of electrochemistry, each metal will reduce the ions of metals below it in the series.
The video below shows an experiment conducted to compare the activity of three metals; zinc, copper and magnesium.


