Oxygen reacts with fluorine to form only #OF_2#, but sulphur which is in the same Group 16 as oxygen, reacts with fluorine to form #SF_2#, #SF_4# and #SF_6#. Why is this?
1 Answer
Sulfur can use its
Explanation:
The diagram below shows the order of orbital energy levels in an atom.
Recall that carbon can form four equivalent orbitals by promoting a
(From CHROMacademy)
In the same way, sulfur can promote its
The ground state electron configuration of
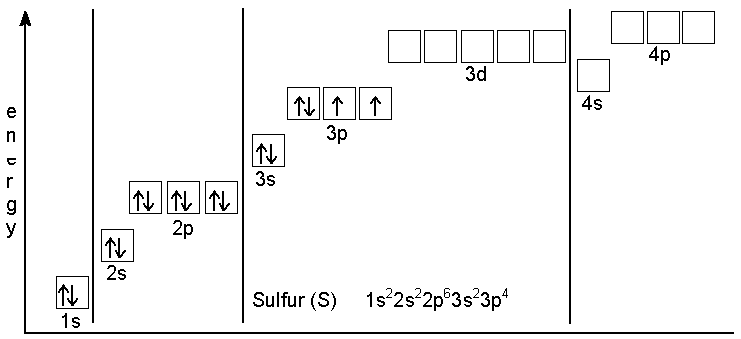
Promoting a
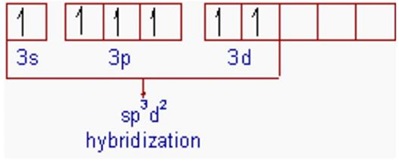
These orbitals can be mixed to form six new hybrid
(From www.alyvea.com)
These six orbitals can then overlap with the half-filled

