Is potassium iodide an ionic or covalent compound?
1 Answer
Potassium iodide is an ionic compound.
Explanation:
Potassium is in Group 1 of the Periodic Table. That means it is a metal.
Iodine is in Group 17 of the Periodic Table. That means it is a nonmetal.
Metals react with nonmetals to form ionic compounds.
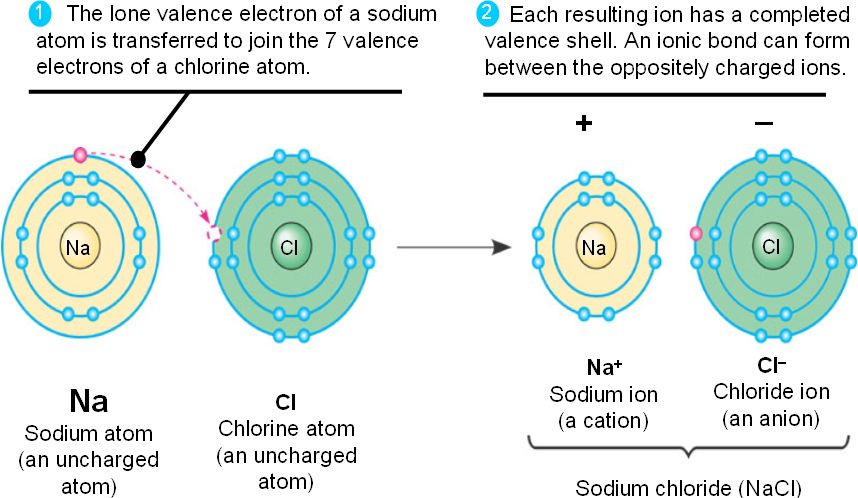
A sodium atom transfers an electron to a chlorine atom to form a sodium ion and a chloride ion. The product is the ionic compound, sodium chloride.
In the same way, a potassium atom transfers an electron to an iodine atom to form a potassium ion and an iodide ion.
So, potassium iodide is an ionic compound.


