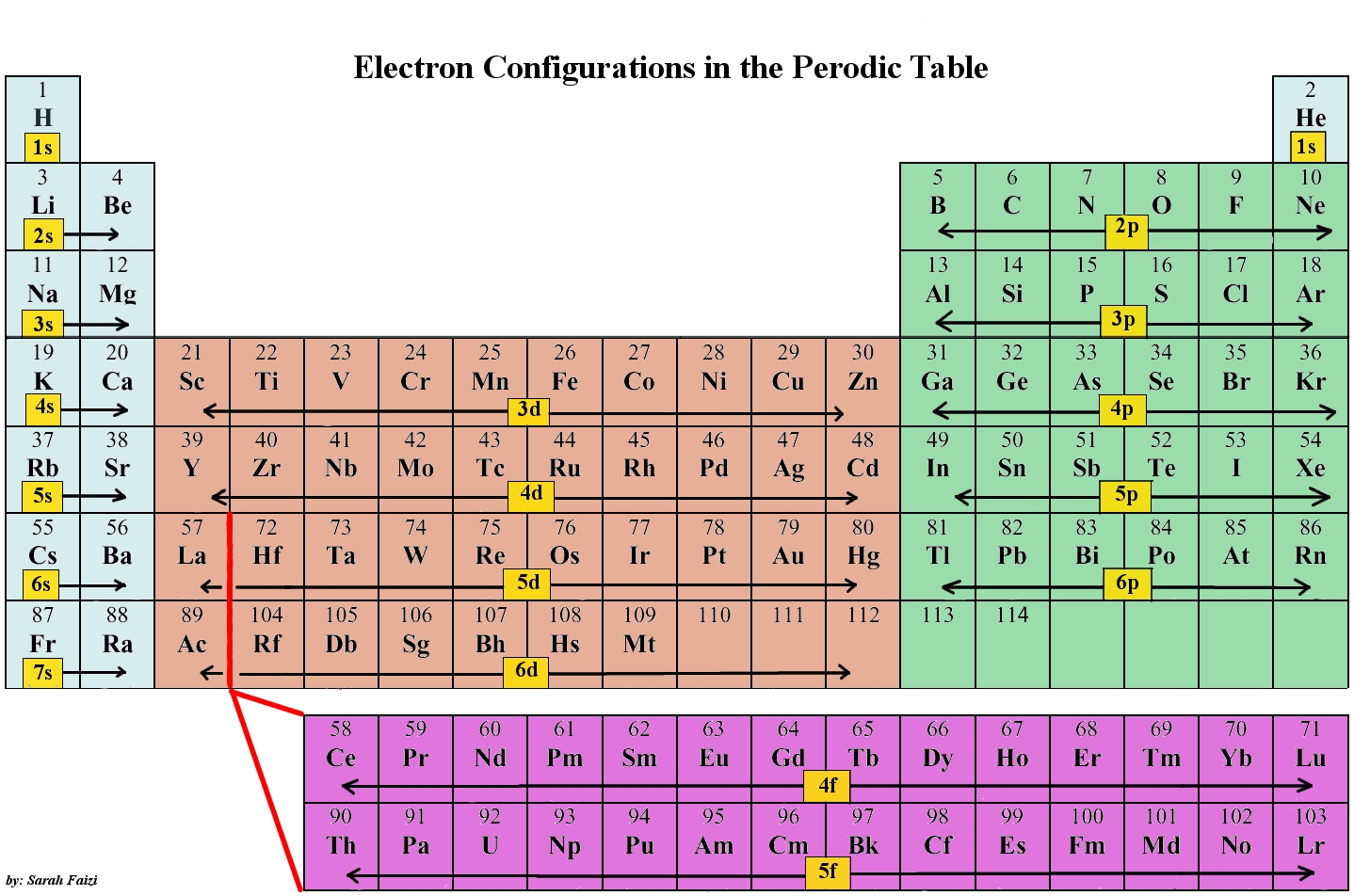
What type of orbitals do actinides and lanthanides mainly use?
1 Answer
Nov 21, 2015
Explanation:
Elements in the lanthanide series often have most of their valence electrons in the