Potassium (#"K"#) is located in group 1, period 4 of the periodic table and has an atomic number of 19.
Since you're dealing with a neutral atom, the number of electrons #"K"# has must equal 19. You could determine how many p-orbitals are occupied in a #"K"# atom by writing its electron configuration
#"K": 1s^(2) 2s^(2) 2p^(6) 3s^(2) 3p^(6) 4s^(1)#
As you can see, the 2p and 3p sublevels each hold six electrons, which means that they are completely occupied. Since every p sublevel has a total of three p-orbitals - #p_x#, #p_y#, and #p_z# - the number of p-orbitals occupied in a #"K"# atom is equal to 6 - 3 p-orbitals on the 2p sublevel and 3 p-orbitals on the 3p sublevel.
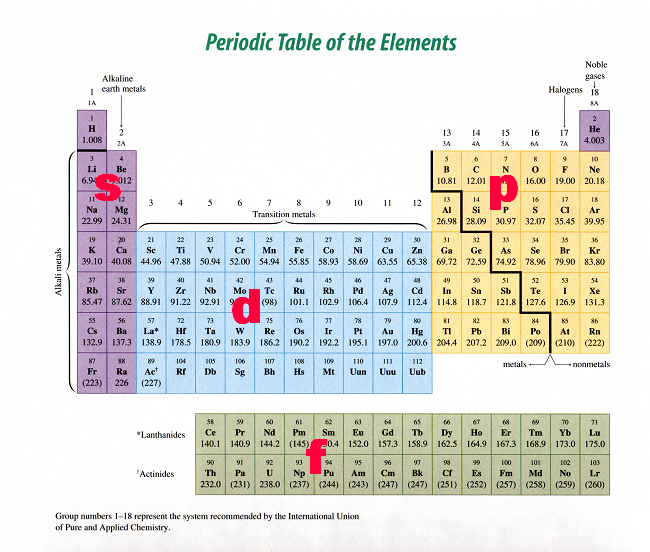
A faster way to figuring out how many p-orbitals are occupied in a #"K"# atom is by looking at the periodic table. The periodic table is split into blocks, as you can see here
 )
)
So, all you have to do is read the periodic table from left to right across each period - much like you would a sentence - until you get to potassium.
If you do this, you'll notice that you've passed two p sublevels: the one that starts at #"B"# and ends at #"Ne"# - the 2p sublevel - and the one that starts at #"Al"# and ends at #"Ar"# - the 3p sublevel. Once again, the conclusion is that #"K"# has 6 p-orbitals which hold 12 of its 19 electrons.
 )
)
 )
) )


