In a piece of metal, what holds the atoms together?
2 Answers
The atoms in a metal are held together by electrostatic forces called metallic bonds.
Explanation:
In a metal like sodium, for example, each

Each atom shares its
You end up with a giant set of molecular orbitals extending over all the atoms.
The result is that the valence electrons are free to move throughout the metal.
The metal atoms that lose their electrons become positive ions, and they are embedded in a "sea" of electrons that is free to move throughout the solid.
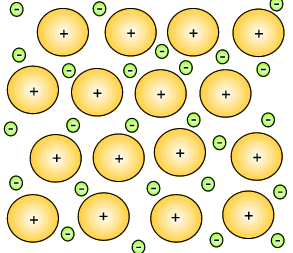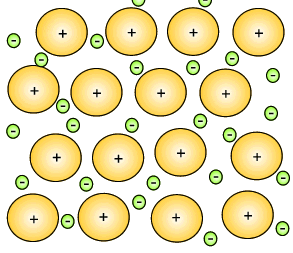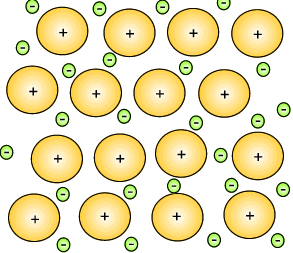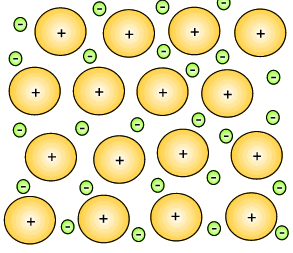
The ions are attracted to the sea of electrons around them, and the electrons are also attracted to them.
These attractive forces hold the metal together in one piece.
We call them metallic bonds.
Explanation:
The standard description of
Because the electrons are non-localized, and the structure is non-molecular, metals tend to be (i) highly
These first 2 properties, which are conferred by metallic bonding, make metals the premier material with which to build tools.
I was to going to include a picture, but I see Ernest has already posted an excellent picture. Apologies for cross posting.


