How does the (R)/(S) method work to convert Fischer projections to bond line views?
1 Answer
A bond-line view gives no stereochemical information. I presume that you want to convert a Fischer projection to a wedge-dash view using (
Step 1. Start with the Fischer projection of D-glucose.
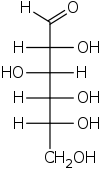
Step 2. Convert this to a "bow tie" view by making all the horizontal bonds into wedges.
Step 3. Assign configurations to the chiral carbons.
The assignments are
Glucose is
Step 4. Draw the wedge-dash structure.
Draw a trial zig-zag structure. Arbitrarily make all the H bonds wedges and the OH bonds dashes.
Some of them will be correct. Others won't.
Assign configurations to the chiral carbons.
The assignments are
We get
This is the wedge-dash structure of glucose.
Step 6. (If you really wanted this) Convert to a bond-line structure.
Omit the H atoms on the chain and convert the wedges and dashes to solid lines.
This structure could represent any of the 16 aldohexoses.

