How does the concentration of an alcohol - water solution affect the volume contraction?
I keep finding information on the effects of temperature, but not on concentration.
Could you also cite sources
THANKS
I keep finding information on the effects of temperature, but not on concentration.
Could you also cite sources
THANKS
1 Answer
DISCLAIMER: LONG ANSWER!
Main source:
Donald A. McQuarrie; Simon, John D.; Physical Chemistry: A Molecular Approach, Viva Student Edition, University Science Books, Sausalito, CA, 2011
The amount of volume contraction or expansion is visible in graphs of total vapor pressure and partial vapor pressures relative to ideality.
Example of negative deviation (volume contraction):
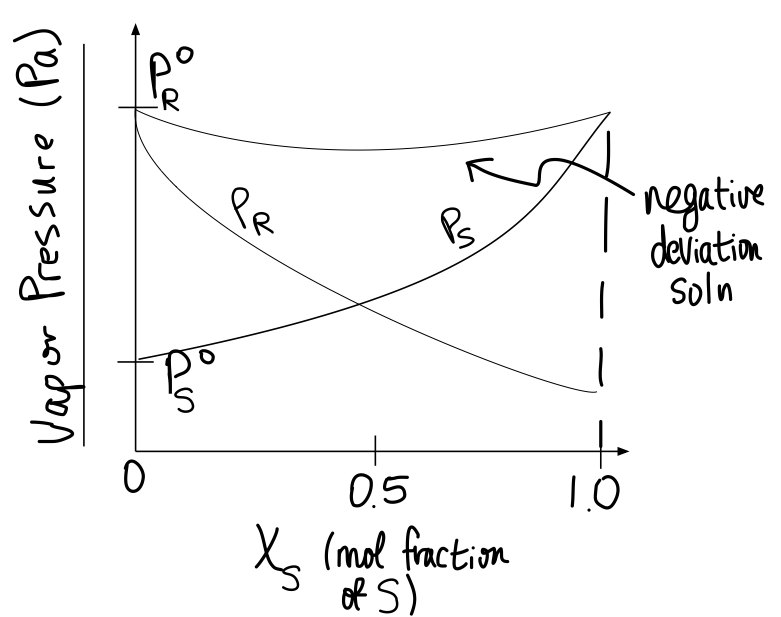
#P^@# is the vapor pressure of the pure substance, and#P_R# is the partial pressure of#R# while mixed with#S# . The top curve is the total vapor pressure.
The greatest volume deviations occur at a
- When there is no volume change after mixing, the total vapor pressure is a constant, in accordance with Raoult's law for ideal solutions.
- When the volume contracts after mixing, the total vapor pressure is smaller than expected (because the molecules interact more strongly, and less liquid at the surface can vaporize).
- When the volume expands after mixing, the total vapor pressure is larger than expected (because the molecules interact less strongly, and more liquid at the surface can vaporize).
This is in general. The effects of concentration (in terms of mol fractions) are shown below in more detail.
WHY VOLUME DEVIATIONS FROM IDEALITY OCCUR
The more similar the intermolecular forces (IMFs) of the solute and solvent, the more easily they mix ("like dissolves like").
When stronger IMFs form between solute and solvent than they each originally had, the volume contracts, and vice versa.
TREND WITH CONCENTRATION (MOL FRACTION SCALE)
For the graphs below:
#chi_1# indicates the mol fraction of#"CS"_2# (#"CHCl"_3# ) in the solution phase in the first (second) graph.#chi_2 = 1 - chi_1# indicates the mol fraction of#"H"_3"C""O""CH"_2"O""CH"_3# (#"H"_3"C"("C"="O")"CH"_3# ) in the solution phase in the first (second) graph.
This is the kind of trend you would see for the alcohols with long alkyl chains (which are hydrophobic):
However, for methanol and ethanol, whose alkyl chains are short (rendering them moderately hydrophilic), you would see something more like this:
In general, it is seen that the greatest effect on vapor pressure (and thus volume deviations) occur closest to a

