How do ionic solutes affect the boiling point?
1 Answer
Ionic solutes raise the boiling point more than nonionic solutes at the same concentration do.
Boiling point elevation is a colligative property. It depends only on the number of particles in the solution.
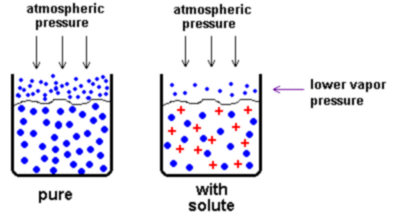
Solute particles are distributed throughout the solution. They "get in the way" of the solvent particles when the solvent wants to evaporate, so we must heat the solution to a higher temperature to make it boil.
The formula for boiling point elevation is
where
The van't Hoff
Non-electrolytes don't dissociate when they dissolve. Thus, one mole of glucose will have one mole of particles in solution, and
NaCl dissociates into Na⁺ and Cl⁻ in water. So if you have 1 mol of NaCl, you'll have 2 mol of particles and i= 2
For CaCl₂, i = 3, for FeCl₃, I = 4, etc.
Thus, a 1 mol/kg solution of FeCl₃ will raise the boiling point of water 4 times as much as a 1 mol/kg solution of glucose.
EXAMPLE
Calculate the boiling point of a 0.15 mol/kg aqueous solution of sodium chloride.
Solution

