How do you know the charge of an element from the periodic table?
1 Answer
Apr 4, 2016
Atoms are neutral, so I'll assume you mean the charges formed when atoms lose or gain electrons to form ions.
Explanation:
Atoms gain or lose valence electrons to become more stable. Metals lose electrons to form positively charged ions and nonmetallic elements gain electrons to form negatively charged ions.
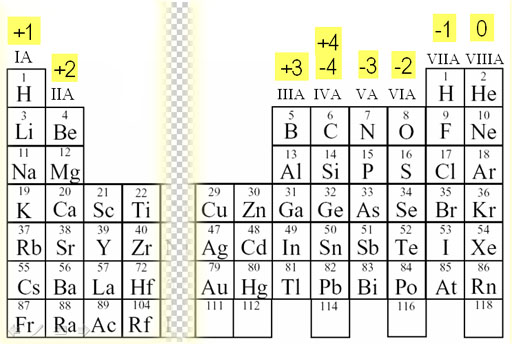
This video discusses how to figure out charges of ions based on their position on the periodic table.
Hope this helps!

