How do you arrange elements' ionic size?
1 Answer
Well, compared to their parent atoms...........
Explanation:
An elemental anion is decidedly LARGER than its parent atom. And an elemental cation is decidedly SMALLER than its parent atom. Why so?
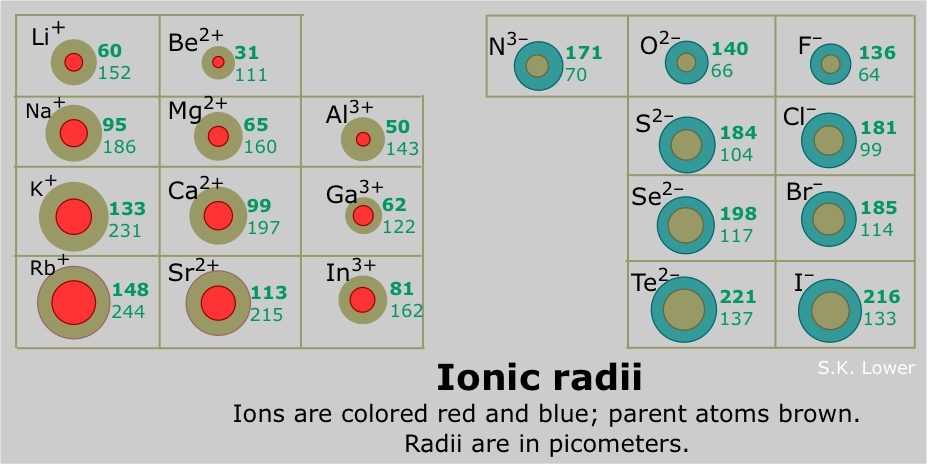
The ionic radii is listed in
On the other hand, the situation is reversed for anions with repsect to their parent atoms. Because the anions are reduced, another electron(s) have been added to the valence shell. This expands the anionic radii because the nuclear charge is still the same and the valence shell expands to accommodate the extra electron(s).......The ionic radius of the nitride ion,

