How do nucleophiles attack the C of the C-O bond?
1 Answer
We can explain this in terms of electrical charges or molecular orbitals.
Explanation:
Electrostatic Explanation
The carbon-oxygen double bond is highly polar.

The nucleophile has a lone pair of electrons and a full or partial negative charge. Let's write it as Nu:⁻.
The lone pair on the Nu:⁻ is strongly attracted to the δ⁺ carbon.
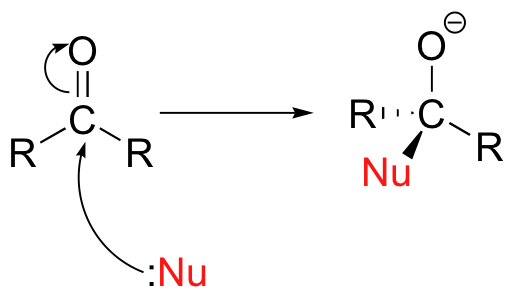
The nucleophile begins to make a coordinate covalent bond.
As the electrons in the π bond move closer towards the oxygen, the O atom becomes increasingly negative.
The movement goes on until the Nu is firmly attached to the C atom, and the O atom has a full negative charge.
Molecular Orbital Explanation
Every molecule has a highest occupied molecular orbital (HOMO) and a lowest unoccupied molecular orbital (LUMO).
A HOMO in one molecule can overlap with a LUMO in another molecule and form a new orbital that is lower in energy.
A carbonyl group has both π and π* orbitals.
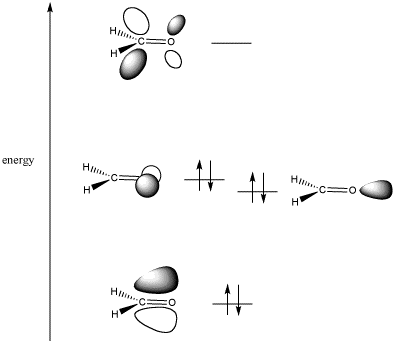
The bonding π orbital has greater electron density on oxygen.
The antibonding π* orbital (the LUMO) has its larger lobe on carbon.
That means that carbon is the “target” for electron donation. The carbonyl carbon is the electrophilic atom.
The HOMO in the nucleophile is the orbital that contains the lone-pair electrons.
The HOMO of the nucleophile approaches the LUMO of the carbonyl carbon at an angle of about 105° to get maximum overlap.

The carbon atom in the adduct ends up with tetrahedral geometry.

