How do metallic bonds share electrons?
1 Answer
Aug 13, 2014
Metallic bonds do not involve the sharing of electrons.
The s and p valence electrons of metals are loosely held. They leave their “own” metal atoms.
This forms a "sea" of electrons that surrounds the metal cations in the solid. The electrons are free to move throughout this electron sea.
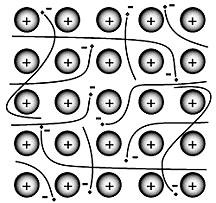
In this model, the valence electrons are free, delocalized, and mobile. The electrons move among all the atoms.


