How do disaccharides dissolve in water?
1 Answer
Jun 5, 2015
Water dissolves disaccharides by forming hydrogen bonds with them.
The simple rule is, "Like dissolves like".
In other words, molecules that are polar will dissolve in a polar solvent like water.
A disaccharide like sucrose has many polar OH groups.

Water molecules are strongly attracted each other by hydrogen bonds.
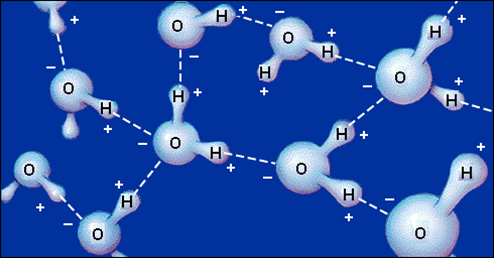
But they are also strongly attracted to the sucrosee.

Water forms hydrogen bonds to the sucrose molecules.

Sucrose can easily get between the water molecules, so sucrose dissolves in water.

