How do covalent bonds hold atoms together?
1 Answer
The attraction between the positively charged nuclei and the negatively charged shared electrons is greater than the repulsions between the nuclei themselves.
Explanation:
Covalent bonds hold atoms together because the attraction between the positively charged nuclei and the negatively charged shared electrons is greater than the repulsions between the nuclei themselves.
As two atoms approach each other, the electrons in their outer shells start to notice the nucleus of the other atom. As a result, they become attracted not only to their own nucleus, but to the nucleus of the other atom as well. The electrons then tend to spend more of their time in between the two nuclei, instead of just around their own nucleus.
The negative electrons spend most of their time between the positive nuclei because of electrostatic attractions. The attractions between the nuclei and the electrons are stronger than the repulsions between the nuclei themselves. The result is a covalent bond with a shared pair of electrons between the two atoms.
In the simple case of two hydrogen atoms, we might represent the process as
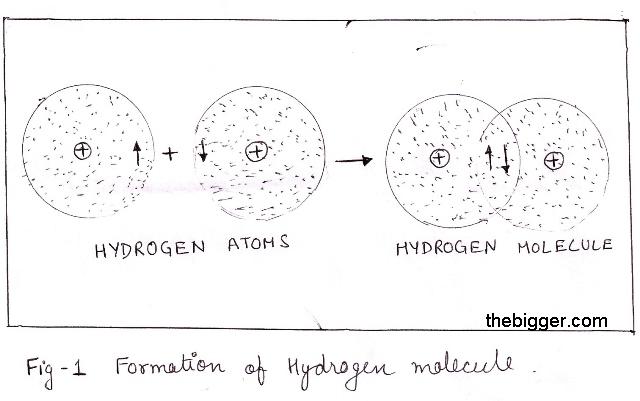
In symbols, we represent the covalent bond as
H•+•H → H:H or H-H
where the shared electrons are represented by a dash or a pair of dots between the two atoms.

