How did Schrodinger's model change Bohr's model of the atom?
1 Answer
Jun 14, 2015
Schrödinger's model changed Bohr's model from one of electrons travelling in fixed orbits to one in which electrons were most likely to be found only in certain regions of space.
Explanation:
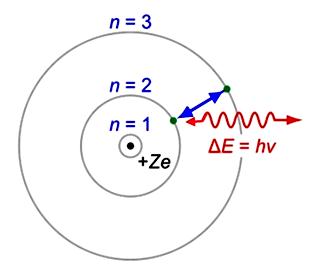
Bohr Model
In the Bohr model, the electrons are particles that occupy only certain orbits of fixed energy around the nucleus.
Schrodinger Model
In the Schrödinger model, the electrons behave as standing waves that have greater probability of being in some regions of space (orbitals) than in others.
The electrons have fixed energies depending on the orbitals they occupy.


