Does CF4 have a polar bond?
1 Answer
Jan 4, 2016
Explanation:
The carbon atom is
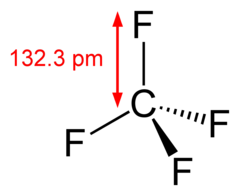
Each
Nevertheless, the
To see why this is so, let's consider the bond dipoles two at a time.
The two
The other two bond dipoles also have a resultant of equal magnitude in the plane of the paper but in the opposite direction.
The two vectors are at 180 ° to each other, and their vector sum is zero.

