Do enantiomers always have an asymmetric carbon?
1 Answer
No, enantiomers do not always have an asymmetric carbon.
Explanation:
Enantiomers are chiral molecules that are non-superimposable mirror images of each other.
Some compounds do not have asymmetric carbon atoms but are still chiral.
If they have two perpendicular planes that are not symmetry planes., and if these planes cannot rotate freely against each other, the compounds are chiral.
Here are some examples:
Cumulenes such as penta-2,3-diene exist as a pair of enantiomers.
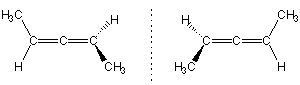
Hindered biphenyls such as 6,6'-dinitro-2,2'-diphenic acid exist as enantiomers because the bulky ortho substituents prevent free rotation about the C-C single bond.

Helices, like screws, are chiral because they can exist in left- or right-handed forms.
Helicenes such as [7]-helicene have steric hindrance at the ends, so they adopt non-planar, enantiomeric helical structures.

A molecule can be chiral if a planar group of atoms is bridged by a chain extending above or below this plane.
Common examples are compounds like 4-bromo[2.2]paracyclophane
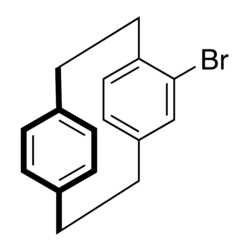
and 2-methylferrocenecarboxylic acid.


