Can you please explain how to use bond energies to determine the change in heat for reactions, or maybe post a link to a video on thermodynamics/ thermochemistry?
1 Answer
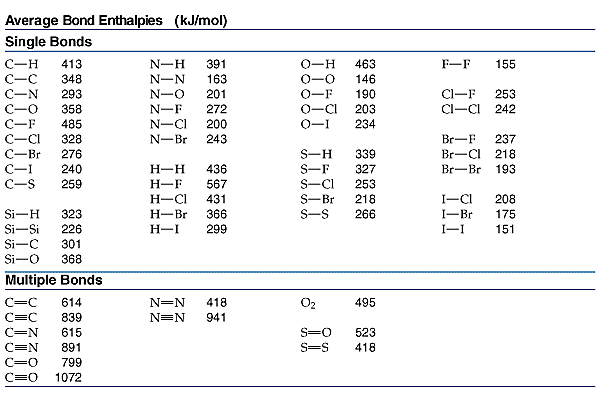
We use Hess's Law when we use bond energies to calculate heats of reaction.
We break all the bonds to form atoms, and then we reassemble the atoms to form new bonds.

For example, in the reaction
we break an H-H bond and a C-Cl bond and form two H-Cl bonds.
We use a table of bond energies like the one below.
and get
The genera formula is
Use bond energies to calculate
We can ignore the C-H bonds, because they are just being broken and re-formed.
Here's a video on the use of bond energies.