Can someone explain the anticlockwise rule for electrode potentials?
All I find online are people asking about the anticlockwise rules in terms of questions they get.
All I find online are people asking about the anticlockwise rules in terms of questions they get.
1 Answer
See below.
Explanation:
The anticlockwise rule is a quick way to determine whether a redox reaction will occur between two species.
Here's how it works.
Example
Will metallic
Solution
Step 1. Write the standard reduction half-reactions for
Make sure that you write the half-reaction with the more negative ( less positive) potential first. This gives
Step 2. Use the anticlockwise rule
Draw anticlockwise curved arrows (the blue arrows in the diagram below) anticlockwise above and below your equations.
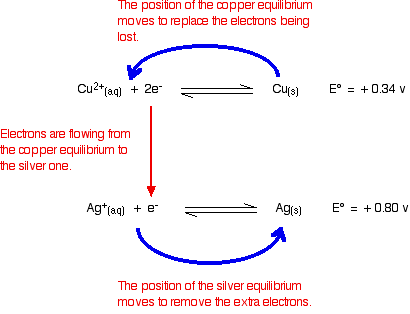
The
Step 3. Determine the equation for the reaction that will occur
We must reverse the top equation.
The rule predicts that
The other conclusion is that

