Are triflate, tosylate and mesylate nucleophilic?
1 Answer
Just the opposite! They are very poor nucleophiles.
A nucleophile is a species that donates a pair of electrons to form a new covalent bond.
A good nucleophile, such as an alkoxide RO⁻, will have a high negative charge on the nucleophilic atom.
The sulfonate ions use resonance to delocalize the negative charge over the rest of the molecule.
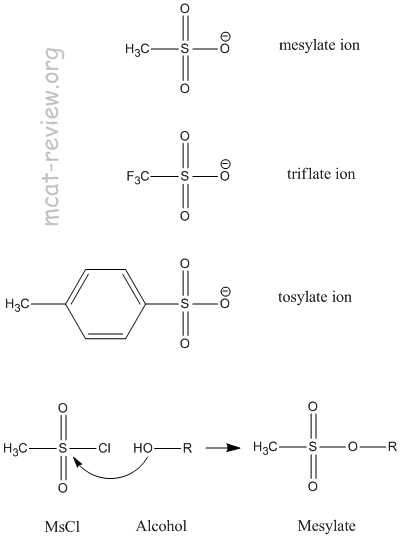
So there is little negative charge on a given O atom.
This makes mesylate, triflate, and tosylate poor nucleophiles, but (for the same reason) they are excellent leaving groups.


