Are SN1 reactions faster in polar solvents?
1 Answer
Yes,
Explanation:
The mechanism of a typical
The transition state for the slow step is
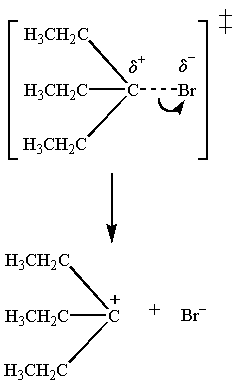
The
Polar protic solvents have large dipole moments. They lower the energy of both the transition state and the starting material.
But they stabilize the transition state more because it is more polar.

This lowers the activation energy, so the reaction goes faster.
The dielectric constant of a solvent is a rough measure of the solvent's polarity. A dielectric constant below 15 is non-polar.
Here is a typical rate order for the same reaction in three different solvents.
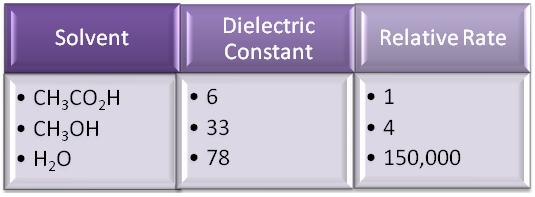
Typical polar protic solvents include water, alcohols, and carboxylic acids.
Here's a video on solvent effects in substitution reactions.

