An electron is in the hydrogen atom with #n = 3#. #|L| = sqrt6 ℏ# . Which is a possible angle between #vecL# and the #z# axis?
1 Answer
The only angle that satisfies the criteria is 65.9°.
Explanation:
In the Schrödinger equation for the hydrogen atom, the squared orbital angular momentum operator,
#bar(ul(|color(white)(a/a) ul(hat(L)^2)Y_(l)^(m_l)(theta,phi) = ul(l(l+1)ℏ^2)Y_(l)^(m_l)(theta,phi) color(white)(a/a)|))" "#
or
#color(blue)(bar(ul(|color(white)(a/a) ul(hat(L)) harr ul(sqrt(l(l+1))ℏ) color(white)(a/a)|)))" "#
where
#l# is the angular momentum quantum number.#Y_(l)^(m_l)(theta,phi)# is the spherical harmonic wave function for the orbital of a given#l# and#m_l# .#m_l# is the magnetic quantum number, and the projection of#l# along the#z# axis.
The
#bar(ul(|color(white)(a/a)ul(hatL_z)Y_(l)^(m_l)(theta,phi) = ul(m_lℏ)Y_(l)^(m_l)(theta,phi)color(white)(a/a)|))" "#
or
#color(blue)(bar(ul(|color(white)(a/a)ul(hatL_z) harr ul(m_lℏ) harr |L|cosθcolor(white)(a/a)|)))" "#
where
#m_l# is the magnetic quantum number and#θ# is the angle that#vecL# makes with the#z# -axis, or the angle of#hatL# with respect to#m_l# .
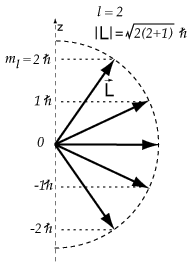
Thus
Some possibilities for
It appears that our electron is a
and
The possible angles for a
#2.4495 color(white)(mml)2color(white)(mml)0.8165color(white)(mll)35.26#
#2.4495 color(white)(mml)1color(white)(mml)0.4082color(white)(mll)65.90#
#2.4495 color(white)(mml)0color(white)(mml)0color(white)(mmmml)90#
#2.4495 color(white)(mm)"-1"color(white)(mm)"-0.4082"color(white)(m)114.09#
#2.4495 color(white)(mm)"-2"color(white)(mm)"-0.8165"color(white)(m)144.74#
The only angle that agrees with those given in the question is 65.90°.


