According to IUPAC, what is the name of #(CH_3CH_2)_3C# #COOH#?
1 Answer
The IUPAC name is 2,2-diethylbutanoic acid.
Let's write the molecule as a bond-line structure.
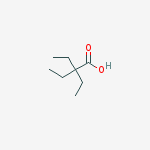
Step 1. Identify the highest-priority functional group.
The only functional group is COOH, so the name ends in -oic acid.
Step 2. Find the longest continuous carbon chain that contains the functional group.
The longest chain contains 4 carbon atoms, so the stem is butan-. This is a butanoic acid.
Step 3. Identify the side chains.
There are two ethyl groups, so this is a diethylbutanoic acid.
Step 4. Locate the side chains.
Number the main chain starting with COOH as C-1. Then both ethyl groups are on C-2.
The IUPAC name is 2,2-diethylbutanoic acid.
Note: Commas join numbers to numbers. Hyphens join numbers to letters. The only space is before the word "acid".

