What interatomic bonding occurs in metals?
2 Answers
Bulk metals are characterized by
Explanation:
....
And metallic bonding results in a plastic, deformable structure, in which the individual metal atoms can move with respect to each other, without disrupting the metallic bond. And as a result, metals are typically (i) malleable, i.e. they can be beaten out into a sheet, and (ii) ductile, they can be drawn out into a wire. And some few metals (which one?) are actually a liquidus...
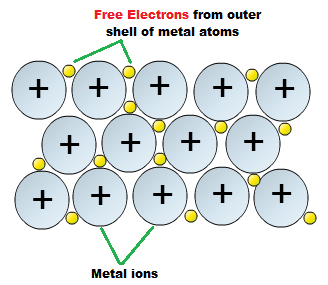
The valence electrons are thus delocalized over the entire lattice, and such interaction, featuring free electrons, is also responsible for (iii) generally good electrical conductivities, and (iv) good thermal conductivities.
A very strong attraction between positive metal ions.
Explanation:
A metallic bond is a VERY strong attraction between positive metal ions that are surrounded by a 'sea' of delocalised electrons.