Question #dfa6b
1 Answer
Here's what I get.
Explanation:
In a silver oxide-zinc battery, the positive electrode (cathode) consists of
The negative electrode (anode) is
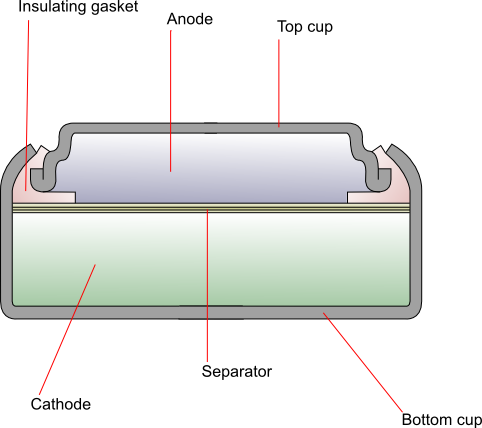
The two half-cells are separated by a permeable combination of layers of plastic membrane, treated cellophane, and absorbent fibres.
The top cup is made of laminated layers of copper, tin, steel, and nickel, and the bottom cup is nickel-plated steel.
An insulating gasket prevents contact between the two cups.
The silver is reduced at the cathode from
The equations for the half-cell reactions and the overall cell reaction are:
At the anode:
At the cathode:
Overall:

