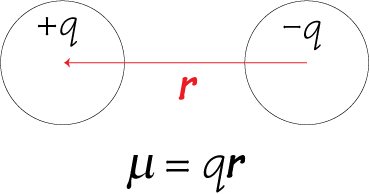
The dipole moment #µ# of a diatomic molecule is a measurement of the separation of two opposite electrical charges.
The magnitude of the moment is equal to the charge #q# multiplied by the distance #r# between them.
#color(blue)(bar(ul(|color(white)(a/a)µ = q × rcolor(white)(a/a)|)))" "#
#"CH"_3"Cl"# and #"CH"_3"F"# are not diatomic molecules, but most of their dipole moment is caused by the polarities of the #"C-Cl"# and #"C-F bonds"#.
Here's a simple argument to account for the differences in dipole moment.
#"F"# is more electronegative than #"Cl"#, but the #"C-F"# bond is only 72 % as long as a #"C-Cl"# bond (139 pm vs 178 pm).
Thus, the charge separation in a #"C-F"# bond would have to be 39 % greater than that in a #"C-Cl"# bond to generate the same dipole moment (#0.72 × 1.39 = 1.00#).
The #"C-F"# bond polarity is not great enough to compensate for the shorter bond distance, so #"CH"_3"F"# has a smaller dipole moment than #"CH"_3"Cl"#.


