Question #9d3bd
1 Answer
Here's what I get.
Explanation:
There are four possible products from the acetylation of ferrocene.
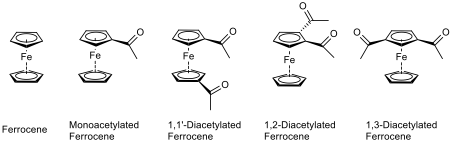
Ferrocene
Ferrocene consists of two cyclopentadienide anions coordinated to an
Its IR spectrum shows no carbonyl absorption.
There are 10 equivalent
This is considerably shielded compared to the normal aromatic range of 6.5 ppm to 9.0 ppm.
However, this is not surprising, given the negative character of the cyclopentadienide rings.
Acetylferrocene
Acetylferrocene has a characteristic carbonyl absorption at
The NMR spectrum shows peaks at 4.19s (
The doublet of
All diacetylferrocenes show strong carbonyl absorption at about
They are distinguished by their NMR splitting patterns.
1,1'-Diacetylferrocene
The compound has 3 proton environments. It shows a
1,2-Diacetylferrocene
This has four proton environments. It shows a
1,3-Diacetylferrocene
This also has four proton environments. It shows

