Question #fc437
1 Answer
Sugar dissolves in water because its
Explanation:
The formula for sucrose is

It contains eight
The oxygen atoms are slightly negative, and the hydrogen atoms are slightly positive.
That is, the
Sucrose molecules are attracted to each other in the crystal because of the dipole-dipole attractions among the
If we add water to sucrose, the
In turn, the sucrose molecules use their
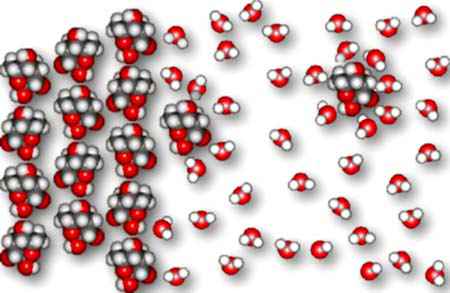
We see below a picture of water molecules attacking the surface of sucrose.

The water molecules surround the sucrose molecules, replacing the sucrose-sucrose
Eventually, the sucrose molecules leave the surface of the crystal and disperse themselves throughout the water as hydrated sucrose molecules.