Question #039d7
1 Answer
The valency of phosphorus is (3.) 3 and 5.
Explanation:
Many chemists define the valency of an element as the number of hydrogen or chlorine atoms that it can combine with.
Phosphorus is in Group 15 of the Periodic Table, so its electron configuration is
It therefore has five valence electrons.
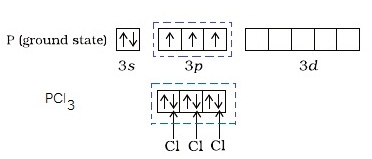
In the orbital diagram above, the three unpaired electrons in the

In this case, the valency of phosphorus is three.
Another possibility is that that
The atom would then have five unpaired electrons, so it could form bonds to five
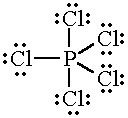
In this case, the valency of phosphorus is five.
Thus, the valency of phosphorus is three and five.

