Question #dda99
1 Answer
Metallic crystals conduct heat electricity and reflects light. It reflects light and conducts electricity because of the same factor but the reasons are different.
Metal atoms lose electrons to form cations and these electrons form an electron cloud around them where the electrons are de-localized.We can think of it as highly polarizable plasma
Metal crystals have positive ions at the lattice positions and the electrons surround it in a cloud. But metal crystals are different from metal atoms because the electrons are not attracted to a particular nucleus and are more free to roam(delocalized) and thus these electrons can jump from the valence band to the conduction band conducting electricity.Metal crystals are denser because though the electrons are loosely packed the metal cations are packed tightly.
The structure of metal crystals

conduction band and valence band
![]()
comparing insulator structures with conductor structures
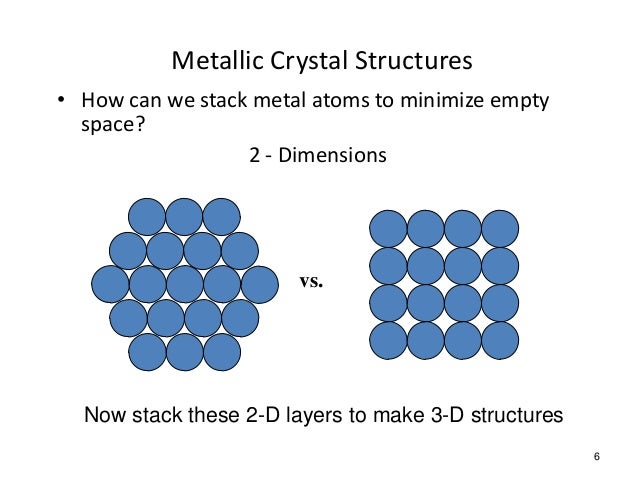
The structure that to the left is the structure of insulator and that to the right is insulator
We see that the space for the electrons to roam is insulators is less than in conductors. But the band gap in both materials is the same.
But in semiconductors the band gaps is large but the structure is same thus if some amount of energy like light and heat, the material will conduct electricity unlike insulators.
So when light or photons come in contact with electrons they readily vibrate and re-emit the light.
Specifically, light with a given frequency causes the electrons to rattle with that same frequency. In the case of visible and infrared light, which are low frequency, the electrons can match the speed and reflect the light.

