Question #4f65c
1 Answer
Here's what's going on here.
Explanation:
The key to this problem lies with the resonance structures of the two molecules, nitric acid,
Now, I will not go into details about how to draw these Lewis structures because I assume that you're familiar with that.
Now, take a look at the three possible resonance structures for the nitrate anion
 )
)
All of these three resonance structures are equivalent, which means that they all contribute equally to the actual structure of the molecule.
Take a look at one of these
- a single bond in the leftmost structure
- a double bond in the middle structure
- a single bond in the rightmost structure
Since all of these structures are equal contributors, you can think of this bond as being a single bond
What actually happens is that the bond between nitrogen and oxygen is a mix between all three cases. This means that you can write
#"Bond" = 1/3 * 1 + 1/3 * 1 + 1/3 * 2 = 2/3 + 2/3 = 1.33#
The bond between nitrogen and oxygen in the nitrate anion is somewhere between a single bond and a double bond, but closer to a single bond.
This is called electron delocalization. The
Now look at the resonance structures for the nitric acid molecule, for which only two major contributors can be drawn
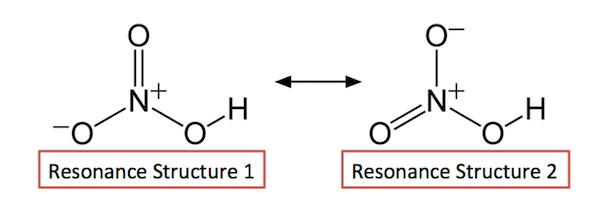
The bond between hydrogen and oxygen prevents electron delocalization for taking place over all three
For the oxygen to which the hydrogen is attached, the bond with nitrogen will always be equal to a single bond.
On the other hand, the other two
#"Bond" = 1/2 * 1 + 1/2 * 2 = 1.5#
Using the time concept again, this happens because these bonds exist halfway between a single bond half of the time, and a double bond the other half of the time.
Remember, in reality the resonance forms do not switch one between the other. All three forms exist simultaneously and they form something called a hybrid structure.
Here's how that would look for the nitrate anion
 )
)
and for the nitric acid molecule
 )
)
So, to sum this up, for the nitrate anion, all three
Remember, a triple bond is shorter and stronger than a double bond, which in turn is stronger and shorter than a single bond.
So the bigger the bond order, the shorter and more stable the bond. Since
The one

